IRB 101
What is an IRB?
IRB is an acronym for Institutional Review Board. The IRB is responsible for the review and approval of all research involving human subjects, as well as scientific validity and ethical review.
What is Research?
In general, research is defined by the Department of Health and Human Services as a “systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge” (45 CFR 46.102(d)).
What is the human subject?
Per Federal regulations (45CFR46.102(f)), a human subject is defined as A living individual about whom an investigator (whether professional or student) conducting research obtains: 1.) Data through intervention or interaction with the individual, or 2.) Identifiable private information.
IRB Policies and Procedures, Manuals, and Other Regulations/Guidance
Find information regarding the IRB’s policies and procedures, manuals, regulations, and guidance.
Where can I find information related to a specific committee?
Each IRB committee has a page with its general description, information.
Find resources related to the committee.
Find the current board member roster for each committee.
Find a detailed listing of meeting deadlines and dates for each committee.
How do I submit a study?
Studies are submitted through our electronic IRB system.
Visit the system, as well as additional information, reference guides, FAQs, log-in guidance, and more.
Is any training required?
Below is a brief overview of the different training requirements. For more detailed information and instructions, please see our Training webpage.
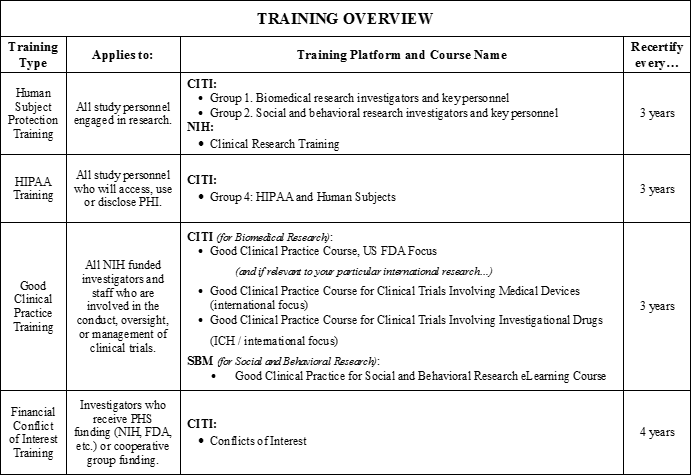
What are the different review options?
Research applications are reviewed at one of three levels, depending on the level of risk to the human participants and on the federal regulations that define the categories of review. The levels of review are exempt, expedited, and full board review. The determination of what level of review is required is determined by the IRB staff, NOT the researcher. Categories of research that can be reviewed via exempt or expedited review can be found at the following links:
To help evaluate the needs for IRB review, please refer to the OHRP decision charts
What are the timelines for review?
IRB approval timeline for minimal risk studies/study submissions:
- Review time for IRB members: 5 business days
- Processing time for IRB Staff: 5 business days
Review timeline for greater than minimal risk studies/study submissions (full board review):
- The study must be submitted before or on the deadline; the study will be reviewed at the meeting associated with the deadline.
- Processing time for IRB staff, after the IRB meeting: 5 business days.
What are the meeting deadlines and dates for submissions to full board?
Check the IRB meeting deadlines and dates.
Users can access both the google calendar for the IRB and/or the detailed listing of deadlines and meeting dates for each committee. Users are able to subscribe to the IRB google calendar. A feed of upcoming events including meeting deadlines and dates can also be viewed on the Main IRB webpage.
When should a study be terminated?
Click on the following link: Termination Information.
Do classroom projects require IRB review?
Before referring to the material below, please make sure you understand the basic definition of research on human subjects (described above).
Please see the following document for more information on classroom activities:
Student-Conducted Research
Find the information regarding Student-Conducted Research.
International Research
International human research refers to research conducted outside the United States using participants from the local community. Such research involving Georgetown University researchers requires review and approval from the GU IRB. Human subjects outside of the United States who participate in research projects conducted or funded by HHS receive the same level of protections as research participants inside the United States.
The approval requirements can be time-consuming, such as translating consent forms, obtaining approval from the international research site, obtaining review and approval by a local ethics review board equivalent to an IRB, etc. Given the additional documentation and review requirements for international research, investigators are strongly encouraged to plan ahead to allow greater time for submitting the protocol application and obtaining IRB approval.
International research applications should identify whether there is a local IRB, Ethics Committee (EC), or government entity that will perform the review in the host country. If a local review has been conducted, a copy of the approval letter/notice should be included in the application. Please consult the International Compilation of Human Research Standards.
